Invasive Species
Invasive species are non-native plants and animals that reproduce and spread quickly, compete with native species for food, degrade habitat, and ultimately cause ecological and economic damages.
Invasive species are perhaps the greatest stressor currently facing the Great Lakes aquatic ecosystem. They are known to modify food webs which alters energy pathways, changes lake productivity, and disrupts fisheries, costing millions of dollars annually in control and mitigation. The Great Lakes has been severely impacted by invasive species, most notably the zebra and quagga mussel, the round goby, and the sea lamprey.
There are more than 185 non-native species in the Great Lakes, but only those species that cause or are likely to cause harm to the economy, environment, or human health are considered invasive.
With our partners at NOAA GLERL, CIGLR is committed to developing information products, predictive models, and strategies to combat and manage invasive species in the Great Lakes region. Our activities in this area include:
1. Forecast Impacts of Invasive Carp and Other Potential Invasive Species
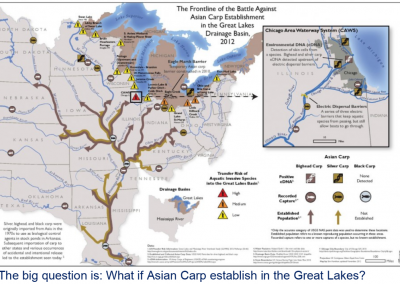
There are about 67 non-native species on the GLANSIS watchlist that pose a threat to the Great Lakes. Some of them, like the invasive carps, have been identified as potential invasive species. Invasive carps include four species, bighead carp, silver carp, grass carp, and black carp. Although they all bear the name carp, grass carp feed on aquatic plants, black carp eat mussels and snails, and bighead and silver carp feed on plankton in the water column. Bighead and silver carp are highly abundant in the Illinois River and have been captured 47 miles away from Lake Michigan. They threaten to invade the Great Lakes and disrupt aquatic food webs and fisheries through their voracious consumption of large volumes of plankton. In river and other lake ecosystems throughout North America, Asia, and Europe, invasive carps have caused a decline in many of the native fish species.
CIGLR has helped produce models for Lakes Huron, Erie, Michigan, and Ontario (in development). The model for Lake Erie shows if invasive carps were to invade, they would dominate the fish community and seriously devalue the vital recreational and commercial fisheries present there. Lake Michigan is colder and has less plankton than the environments where they currently exist. But, accounting for diet flexibility and subsurface habitat in the model, the research team demonstrated that nearly all of Lake Michigan contains suitable habitat for bigheaded carp. Habitats with greatest potential to support bigheaded carp were located near river mouths and in Green Bay, which agrees with previous studies. However, their research also demonstrates that Lake Michigan’s offshore areas are suitable for bigheaded carp. Although offshore areas offer a relatively low-quality habitat, making them less appealing for resident populations, they may provide migration corridors through which bigheaded carp could spread to more food-rich areas in the lake. Lake Michigan’s vulnerability to these fishes will likely continue to increase as climate change progresses or if nutrient pollution increases, despite a competitive feeding pressure from dreissenid mussels on the plankton communities.
Invasive carp impacts are likely driven by lake productivity and fish community structure, thus potentially impacting each of the Great Lakes differently. In our most recent study, we developed a Lake Ontario EwE model and initiated a collaboration network with scientists at USGS Great Lakes Center, Cornell University, Department of Fisheries and Oceans Canada, Ministry of Natural Resources and Forestry (Canada), SUNY Buffalo State College, NYSDEC, and Toronto and Region Conservation Authority. The Ecopath with Ecosim (EwE) food web model to quantify the potential impacts of invasive carp to the Lake Ontario and lower Illinois River food web and fisheries. Preliminary results for bigheaded carp impacts on the Lake Ontario food web showed bigheaded biomass could reach 4.6 tonne/km2, or 34% of the total fish biomass at the end of simulation.
We plan to continue developing similar ecosystem models to assess the invasive carp threat in the other Great Lakes and their embayments. Since the Great Lakes each have unique characteristics, the potential growth, survival, and impacts of invasive carps are expected to be different from those predicted for Lake Erie, Lake Michigan, and Lake Ontario. This information is urgently needed to support informed management decisions regarding invasive carp control and to identify the resident species at risk for impacts by a silver and bighead carp invasion. CIGLR is also using these models to predict ecological impacts on the Great Lakes food webs by other invasive species from the watchlist, such as killer shrimp and golden mussels.
2. Dreissenid Mussels and HABs

Click image to read and watch 13 ABC’s interview with NOAA GLERL and CIGLR about how mussels “spit out’ toxic microcystin and play a part in Lake Erie algae.
Invasive mussels in Lake Erie may be contributing to the lake’s harmful algal bloom (HAB) problem, according to scientists from Central Michigan University, University of Michigan, CIGLR, and the National Oceanic and Atmospheric Administration (NOAA) Great Lakes Environmental Research Laboratory (GLERL). After three decades of water quality improvements in Lake Erie, the recent proliferation of HABs has motivated research teams to better understand the role that zebra and quagga mussels play in the lake. Results from a recent series of laboratory experiments suggest that the plankton in Lake Erie provide invasive mussels with enough food that they can reject the not-so-appetizing and less nutritious HAB species Microcystis. This research team completed 10 trials of two-week laboratory experiments to measure mussel grazing on intact plankton assemblages from Lake Erie. In all 10 experiments, mussels fed heavily on small algae, diatoms, and larger protists, while Microcystis and some colonial green algae were left uneaten. The implication of this selectivity is that conventional approaches to measuring phytoplankton biomass and community composition are inadequate for studying the impacts of mussels on the lower food web. Instead, future studies will need to leverage ‘omics approaches to quantify the impacts of these invaders. This result is critical for the management of HABs in the Great Lakes because it shows that nutrient availability is not the only factor that leads to success of Microcystis.
3. Omics and eDNA
The Cooperative Institute for Great Lakes Research (CIGLR) in partnership with NOAA-GLERL has been working to develop genomic tools that will advance conservation biology and biodiversity management. For successful management of bio-invasions and monitoring the reintroduction of threatened or endangered species in the Great Lakes or elsewhere, managers and stakeholders need timely and robust scientific advice.

Dr. Nate Marshall sampling eDNA and eRNA from a mesocosm experiment. Click image to learn more. Photo Credit: Aubrey Lashaway.
The Laurentian Great Lakes are one of the most rapidly changing ecosystems in the world in large part due to more than 185 species invasions over the past two centuries. The Lakes are swiftly undergoing changes in temperature, hypoxia, and invasive species representations, whose effects are especially acute in warmer productive areas that are important to fisheries, e.g., western Lake Erie and Saginaw Bay in Lake Huron. Arguably the most radical change has been and continues to be mediated by invasive Dreissenid mussels (Dreissena polymorpha (zebra mussel) and D. bugensis (quagga mussel)). Dreissenids radically change community structure and the flow of energy and matter in most systems they invade. In offshore regions, they starve the pelagic food web by sharply reducing phytoplankton biomass, causing zooplankton and fish population declines and dietary shifts from phytoplankton to littoral benthic algae. In contrast, in nearshore regions invasive Dreissenid mussels promote nuisance benthic algal growth and harmful cyanobacterial blooms by selective removal of non-cyanobacterial phytoplankton. For Microcystis, which often dominates harmful algal blooms in the Great Lakes, observations indicate that a trade-off between adaptation to nutrient levels and grazing resistance may exist. Nutrient levels, in particular nitrogen levels and N:P ratios, also influence toxin levels produced by Microcystis-dominated blooms, adding additional complexity to the regulation of key Microcystis traits that affect its ecosystem-levels impacts.
Traditionally, scientists have relied on conventional approaches such as visual surveys to try and determine the dynamic responses of entire lake biological communities to ongoing physical and chemical changes in key regions of the Great Lakes. However, low rates of species detection can increase the cost and labor required for this approach. During the last decade, eDNA has become a powerful tool used to collect information from aquatic habitats. eDNA methodology is cost-effective and easier to collect compared to traditional sampling, and has improved the management and assessment of a species’ distribution. eDNA analysis has been incorporated into Great Lakes monitoring programs for detection of a wide-range of organisms, including species that are endangered/threatened, invasive, or economically important.
The team has developed a new methodology that uses environmental DNA and RNA (eDNA and eRNA) to accurately detect and distinguish the presence of living organisms and improve estimators of their relative abundances. eDNA and eRNA refer to genetic material naturally released from an organism into the environment, through feces/urine, mucus, gametes, skin/tissue, carcasses, and so on. It can be collected from the environment (such as lake water), rather than directly from an organism, and analyzed to identify the species present. Because the concentration of eRNA degrades much faster than the eDNA, it provides a predictor for estimating time since genomic material was released from an organism into the environment, thus improving spatiotemporal estimates of community composition. This new method is easily modified and can be applied to monitoring different species within the Great Lakes or elsewhere in the world. The researchers will apply this method to examine how Dreissenid mussels alter the composition of cyanobacterial communities, and more specifically the composition of Microcystis populations. It is a powerful technique that will enhance the confidence of rapid decision-making by environmental managers and stakeholders and expand monitoring of ecosystem health.
4. Great Lakes Aquatic Nonindigenous Species Information System (GLANSIS)
The Great Lakes Aquatic Nonindigenous Species Information System (GLANSIS) is a searchable regional database with species-specific fact sheets, threat assessments, and maps designed to improve public education and inform prevention, management, and control of invasive and non-native species. The database is a collaboration between Michigan Sea Grant, NOAA, Michigan DNR Institute for Fisheries Research, USGS, EPA, and CIGLR. CIGLR has helped the partnership by maintaining current data, making updates when needed, as well as improving the database through technologies and products. This has involved programming enhancements for improved functionality, adding reports of non-native species in new locations, adding new species to the watchlist of likely invaders, moving species from the watchlist to the established category, and adding maps habitat suitability for non-native species. The Sea Grant Great Lakes Network works to ensure the most current state of knowledge regarding current and future threats is being delivered to the public and managers. Information served through GLANSIS helps managers make informed decisions when formulating and implementing strategies to prevent, control, and mitigate the introduction and impacts of invasive and non-native species.
Stay up-to-date on the most recent news and scientific media generated from our Invasive Species research here:
Products & Resources
- GLANSIS database and information (website): Great Lakes Aquatic Nonindigenous Species Information System (GLANSIS) is a searchable database with fact sheets, threat assessments, and maps designed to improve stakeholder education, and inform prevention, management, and control of aquatic nonindigenous species (ANS).
- GLANSIS (poster)
- GLANSIS Watchlist (poster)
- Invasive Species (CIGLR factsheet)
- Aquatic Invasive Species Research at NOAA GLERL (NOAA GLERL factsheet)
- How Would and Asian Carp Invasion Affect Fish in Lake Erie? (NOAA GLERL infographic)
- Invasive Mussels and the Productivity of Lake Michigan (NOAA GLERL infographic)
- Lake Michigan Food Web (poster)
- Lake Huron Food Web (poster)
- Lake Ontario Food Web (poster)
- Lake Superior Food Web (poster)
- Lake Erie Food Web (poster)
- Invasive Species Research (bookmark)
- Mussel Research (bookmark)
- Dreissenid Mussel Crossword Puzzle (game)
- Great Lakes Food Web Information (trivia)
- Invasive Carp Information (trivia)
News
- Using Environmental DNA and RNA to Inform Biomonitoring and Bioassessment Management Plans, CIGLR 2019 Fall eNews, 12/2019
- Great Lakes Water Life Database Documents Biodiversity of Great Lakes Native Species, MSU Extension, 8/21/2019
- Large Lakes Worldwide Share Many of the Same Threats, Great Lakes Echo, 8/15/2019
- A Mussel Poop Diet Could Fuel Invasive Carp’s Spread Across Lake Michigan, Science News, 8/13/2019
- GLANSIS: A Database of Aquatic Invaders for Scientists and Citizens Alike, CIGLR 2018 Spring eNews, 06/2018
- GLANSIS: A ‘one-stop shop’ for information on aquatic invaders, Michigan State University Extension, 2/25/2018
- Alien language: Stages of invasion, Michigan State University Extension, 2/24/2019
- Alien language: Understanding terminology about nonindigenous species, Michigan State University Extension, 2/24/2019
- Quagga Mussels and Nutrients Linked to Lake Michigan Productivity Loss, CIGLR 2017 Fall eNews, 12/2017
- Invasive Hitchhikers: Catching a Ride on Lake Currents; CIGLR 2017 Spring eNews, 06/2017
- Modelling the Spread of Aquatic Invasive Species in Great Lakes; Water Canada, 5/29/2017
- Collaborative team identifies 16 high-risk Great Lakes invaders; NOAA Great Lakes Environmental Research Laboratory’s Blog, 12/2/2016
Asian Carp
- Climate Change Would Limit Competition For Asian Carp If Introduced In Lake Michigan, Study Finds, Wisconsin Public Radio, 7/8/2020
- Warming Could Lower One Barrier to Invasive Fish Reaching Great Lakes, Scientific American, 7/8/2020
- U of M study: Climate change may aid Asian carp invasion of Great Lakes, Michigan Radio, 7/8/2020
- Climate warming increases Asian carp threat to Lake Michigan by offsetting quagga mussel ‘ecological, phys.org, 7/8/2020
- Climate warming increases Asian carp threat to Lake Michigan by offsetting quagga mussel ‘ecological barrier’, Michigan News, 7/7/2020
- Global warming could help Asian carp invade Lake Michigan, Earth.com, 7/7/2020
- Modeling Lake Michigan’s Suitability for Asian Carp, CIGLR 2020 Summer eNews, 09/2020
- Bigheaded carp pose big threat, new model suggests, Great Lakes Echo, 6/5/2020
- Big Fish Story: Asian carp are poised to enter Lake Michigan, but to what extent is a matter of debate. Rackham alumnus Peter Alsip’s research sheds light on the watery invaders’ potential whereabouts, Discover Rackham, 1/22/2020
- Asian Carp Capable of Surviving in Much Larger Areas of Lake Michigan Than Previously Thought, University of Michigan Faculty News, 11/22/2019
- Favorite Food Not on the Menu? No Problem for Asian Carp – They Eat Almost Anything, Great Lakes Echo, 8/21/2019
- Lake Michigan’s ‘Plankton Desert’ No Problem for Bighead Carp, Peninsula Pulse, 8/16/2019
- Study: Asian Carp Could Thrive in Lake Michigan, Despite Earlier Doubts, Bridge, 8/12/2019
- U-M study: Asian Carp Capable of Surviving in Much Larger Areas of Lake Michigan than Previously Thought, Michigan News, 8/12/2019
- Study: Asian Carp Could Expand Across Greater Area of Lake Michigan than Previously Thought, Michigan Radio, 8/12/2019
- Asian Carp have Never Breached a Body of Freshwater the Size of Lake Michigan. Here’s the Bizarre Way They Could Survive and Thrive in the World’s Fifth Largest Lake, Chicago Tribune, 8/12/2019
- Asian Carp Could be a lot Worse to the Great Lakes than Previously Thought, UofM Study Shows, Fox 2 Detroit, 8/12/2019
- New Study Finds Asian Carp Could Survive Throughout Lake Michigan, Wisconsin Public Radio, 8/12/2019
- Are the Great Lakes Next? Video Shows Hundreds of Asian Carp Shocked in Kentucky Lake, Fox 2 Detroit, 8/1/2019
- Can Asian Carp Survive in Lake Michigan?, CIGLR 2019 Spring eNews, 06/2019
- Asian carp would damage fish species in Lake Erie; Capital News Service, 2/5/2016
- Ecological casualties: winners and losers in the war on carp; Great Lakes Echo, 1/28/2016
- All-you-can-eat Asian carp are gluten free; The Times Herald, 1/28/2016
- Forecasting Future of Asian Carp In Great Lakes: An Erie Story; Medill News Service, 1/13/2016
- Asian Carp Would Significantly Alter – But Not Destroy – Lake Erie Fisheries; WaterNews, 1/7/2016
- Impact of Asian carp on Great Lakes could be overestimated; The Michigan Daily, 1/7/2016
- Report: Asian carp could become dominant Lake Erie fish; Sandusky Register, 1/6/2016
- ASIAN CARP COULD CLAIM ONE-THIRD OF LAKE ERIE BIOMASS; Newsweek, 1/6/2016
- Lake Erie Asian carp: How great a threat to Great Lakes?; Christian Science Monitor, 1/5/2016
- Invasive Asian carp could overtake Lake Erie, study finds; Washington Post, 1/5/2016
- Asian Carp Could Dominate Lake Erie, Study Says; The Weather Channel, 1/5/2016
- Researchers Forecast Impact Of Invasive Asian Carp On Lake Erie’s Native Fish Species; Tech Times, 1/5/2016
- LOCAL Report sounds alarm of Asian carp threat; Toledo Blade, 1/5/2016
- Asian carp could cause some Lake Erie fish to decline, others to increase; Michigan News, 1/4/2016
- Study: Asian carp could develop huge presence in Lake Erie; The Seattle Times, 1/4/2016
- Asian carp would dominate Lake Erie biomass, study finds; Milwaukee Journal Sentinel, 1/4/2016
- Study: Walleye, trout will be hardest hit by Asian carp; Detroit News, 1/4/2016
- Asian Carp Invasion Of Lake Erie Would Probably Have Little Impact On Fish Biomass; Headline and Global News, 8/9/2014
- Experts doubt Asian carp invasion would harm Lake Erie fishery; Milwaukee-Wisconsin Journal Sentinel, 8/8/2014
Publications
Alsip, P.J., H. Zhang, M.D. Rowe, D.M. Mason, E.S. Rutherford, C.M. Riseng and Z. Su. 2019. Lake Michigan’s Suitability for Bigheaded Carp: The Importance of Diet Flexibility and Subsurface Habitat. Freshwater Biology. (DOI:10.1111/fwb.13382). [Altmetric Score]
Alsip, P.J., H. Zhang, M.D. Rowe, E. Rutherford, D.M. Mason, C. Riseng and Z. Su. 2020. Modeling the interactive effects of nutrient loads, meteorology, and invasive mussels on suitable habitat for Bighead and Silver Carp in Lake Michigan. Biological Invasions. (DOI:10.1007/s10530-020-02296-4). [Altmetric Score]
Beletsky D. , R. Beletsky, E.S. Rutherford , J.L. Sieracki, J.M. Bossenbroek, W.L. Chadderton, M.E. Wittmann, G. Annis and D. Lodge. 2017. Spread of aquatic invasive species by lake currents. Journal of Great Lakes Research. 43:14-32. (DOI:10.1016/j.jglr.2017.02.001). [Altmetric Score]
Cooke, R.M., M.E. Wittmann, D.M. Lodge, J.D. Rothlisberger, E.S. Rutherfod, H. Zhang and D.M. Mason. 2014. Out-of-sample validation for structured expert judgment of Asian Carp establishment in Lake Erie. Integrated Environmental Assessment and Management. 10(4):522-528. (DOI:10.1002/ieam.1559).
Davidson, A.D., A.J. Fusaro, R.A. Sturtevant, E.S. Rutherford and D.R. Kashian. 2017. Development of a risk assessment framework to predict invasive species establishment for multiple taxonomic groups and vectors of introduction. Management of Biological Invasions. 8:25-36. (DOI:10.3391/mbi.2017.8.1.03).
Denef, V.J., H.J. Carrick, J.F. Cavaletto, E. Chiang, T.H. Johengen and H.A. Vanderploeg. 2017. Lake Bacterial Assemblage Composition Is Sensitive to Biological Disturbance Caused by an Invasive Filter Feeder. American Society for Microbiology: mSphere. 2(3). (DOI:10.1128/mSphere.00189-17). [Altmetric Score]
Fusaro, A.J., E. Baker, W. Conrad, A. Davidson, K. Dettloff, J. Li, G. Núñez-Mir, R.A. Sturtevant and E.S. Rutherford. A risk assessment of potential Great Lakes aquatic invaders. NOAA Technical Memorandum GLERL-169.
Kao, Y-C., S. Adlerstein and E.S. Rutherford. 2016. Assessment of bottom-up and top-down controls on the collapse of alewives Alosa pseudoharengus in Lake Huron. Ecosystems. 19:803-831. (DOI:10.1007/s10021-016-9969-y). [Altmetric Score]
Lodge, D.M., P.W. Simonin, S.W. Burgiel, R.P. Keller, J.M. Bossenbroek, C.L. Jerde, A.M. Kramer, E.S. Rutherford, M.A. Barnes, M.E. Wittmann, W.L. Chadderton, J.L. Apriesnig, D. Beletsky, R.M. Cooke, J.M. Drake, S.P. Egan, D.C. Finnoff, C.A. Gantz, E.K. Grey, M.H. Hoff, J.G. Howeth, R.A. Jensen, E.R. Larson, N.E. Mandrak, D.M. Mason, F.A. Martinez, T.J. Newcomb, J.D. Rothlisberger, A.J. Tucker, T.W. Warziniack and H. Zhang. 2016. Risk analysis and bioeconomics of invasive species to inform policy and management. Annual Review of Environment and Resources. 41:453-88. (DOI:10.1146/annurev-environ-110615-085532). [Altmetric Score]
Marshall, Nathaniel T., Henry A. Vanderploeg & Subba Rao Chaganti. 2021. Environmental (e)RNA advances the reliability of eDNA by predicting its age. Scientific Reports. 11:2769. (DOI: 10.1038/s41598-021-82205-4). [Altmetric Score]
Nalepa, T.F. 2014. Relative comparison and perspective on invasive species in the Laurentian and Swedish Great Lakes. Aquatic Ecosystem Health and Management. 17(4):394-403. (DOI:10.1080/14634988.2014.972494).
Reisinger, L.S., A.K. Elgin, K.M. Towle, D.J. Chan and D.M. Lodge. 2017. The influence of evolution and plasticity on the behavior of an invasive crayfish. Biological Invasions. 19(3):815-830. (DOI:10.1007/s10530-016-1346-4). [Altmetric Score]
Rowe, M.D., E.J. Anderson, H.A. Vanderploeg, S.A. Pothoven, A.K. Elgin, J. Wang and F. Yousef. 2017. Influence of invasive quagga mussels, phosphorus loads, and climate on spatial and temporal patterns of productivity in Lake Michigan: A biophysical modeling study. Limnology and Oceanography. (DOI:10.1002/lno.10595). [Altmetric Score]
Smith, J.P., E.K. Lower, F.A. Martinez, C.M. Riseng, L.A. Mason, E.S. Rutherford, M. Neilson, P. Fuller, K.E. Wehrly and R.A. Sturtevant. 2019. Interactive mapping of nonindigenous species in the Laurentian Great Lakes. Management of Biological Invasions. 10(1):192-199. (DOI:10.3391/mbi.2019.10.1.12).
Sturtevant, R.A., L. Berent, T. Makled, A.J. Fusaro and E.S. Rutherford. 2016. An overview of the management of established nonindigenous species in the Great Lakes. NOAA Technical Memorandum GLERL-168.
Sturtevant, R.A., J. Larson, L. Berent, M. McCarthy, A. Bogdanoff, A.J. Fusaro and E.S. Rutherford. 2014. An Impact Assessment of Great Lakes Aquatic Nonindigenous Species. NOAA Technical Memorandum GLERL-161.
Sturtevant, R., D.M. Mason, E.S. Rutherford, A. Elgin, E. Lower and F. Martinez. 2019. Recent history of nonindigenous species in the Laurentian Great Lakes; An update to Mills et al., 1993 (25 years later). Journal of Great Lakes Research. (DOI:10.1016/j.jglr.2019.09.002). [Altmetric Score]
Tucker, A.J, W.L. Chadderton, C.L. Jerde, M.A. Renshaw, K. Uy, C. Gantz, A.R. Mahon, A. Bowen, T. Strakosh, J.M. Bossenbroek, J.L. Sieracki, D. Beletsky, J. Bergner and D.M. Lodge. 2016. A sensitive environmental DNA (eDNA) assay leads to new insights on Ruffe (Gymnocephalus cernua) spread in North America. Biological Invasions. 18:3205-3222. (DOI:10.1007/s10530-016-1209-z). [Altmetric Score]
Wang, L., C.M. Riseng, L.A. Mason, K.E. Wehrly, E.S. Rutherford, J.E. McKenna, Jr., C. Castiglione, L.B. Johnson, D. Infante, S.E. Sowa, M. Robertson, J. Schaeffer, M. Khoury, J. Gaiot, T. Hollenhorst, C. Brooks and M. Coscarelli. 2015. A spatial classification and database for management, research, and policy making: The Great Lakes aquatic habitat framework. Journal of Great Lakes Research. 41:584-596. (DOI:10.1016/j.jglr.2015.03.017). [Altmetric Score]
Wittman, M.E., G. Annis, A.M. Kramer, L. Mason, C. Riseng, E. S. Rutherford, W. L. Chadderton, D. Beletsky, J.M. Drake and D.M. Lodge. 2016. Refining species distribution model outputs using landscape scale habitat data: Forecasting Grass Carp and Hydrilla verticillata establishment in the Great Lakes Region. Journal of Great Lakes Research. 43:298-307. (DOI:10.1016/j.jglr.2016.09.008). [Altmetric Score]
Wittmann, M., R. Cooke, J. Rothlisberger, E.S. Rutherford, H. Zhang, D.M. Mason and D. Lodge. 2014. Use of structured expert judgment to forecast invasions by Bighead and Silver carp in Lake Erie. Conservation Biology. 29(1):187-197. (DOI:10.1111/cobi.12369). [Altmetric Score]
Zhang, H., E. Rutherford, D.M. Mason, J. Breck, R. Cooke, M. Wittmann, T. Johnson, X. Zhu and D. Lodge. 2016. Forecasting the Impacts of Silver and Bighead Carp on the Lake Erie Food Web. Transactions of the American Fisheries Society. 145(1):136-162. (DOI:10.1080/00028487.2015.1069211). [Altmetric Score]
PrincipaI Investigator(s):
Casey Godwin (CIGLR)
Hunter Carrick (CMU)
Vincent Denef (UM)
Yu-Chun Kao (MSU)
Rochelle Sturtevant (MSU)
NOAA Technical Lead(s):
Felix Martinez (NOAA NOS)
Invasive Species Photo Gallery
Michele Wensman (CIGLR) measures and prepares invasive Dreissenid mussel specimens. Photo Credit: CIGLR.
Zebra mussels are native to the Black and Caspian seas of Western Asia and Eastern Europe and have spread across the world via shipping. Zebra mussels are invasive species to the Great Lakes and were first found in this system in 1988 when they were discovered in Lake St. Clair and the Detroit River. Photo Credit: D. Jude, University of Michigan.
Sea lampreys (Petromyzon marinus) entered the Great Lakes system in the 1800s through manmade locks and shipping canals. They were first discovered in Lake Ontario in 1835. Sea lamprey prey on commercially important fish species, such as lake trout, living off of the blood and body fluids of adult fish. During its life as a parasite, each sea lamprey can kill 40 or more pounds of fish. Photo Credit: T. Lawrence, Great Lakes Fishery Commission.
Quagga mussels are native to eastern Europe and invasive to the Great Lakes. THey were first found in the Great Lakes in 1989 near Port Colbourne in Lake Erie. Quagga mussels are contributing to the dramatic decline of the shrimp-like amphipod known as Diporeia, a key food source for a number of native Great Lakes fish at various points in the food web; and they are contributing to reemergence of blooms – the toxic alga known as Microcystis – around the lakes. Photo Credit: Michele Wensman.
Invasive carp, bighead carp and silver carp, were first brought to the southern United States to aid in the cleaning of fish hatcheries. Due to flooding and hatchery overflow, invasive carp were accidentally released into waterways and have now been flourishing in the Mississippi River basin for nearly 40 years. They compete with native fish by devouring the planktonic food, the microscopic plants and animals at the base of the food web, causing extreme stress and ultimately an environment where the native fish struggle to survive. Photo Credit: D. O’Keefe, Michigan Sea Grant.
When frightened, Bighead and Silver varieties of invasive carp can jump up to 10 feet out of the water. Photo Credit: AP Photo/Illinois River Biological Station via the Detroit free Press, Nerissa Michaels.
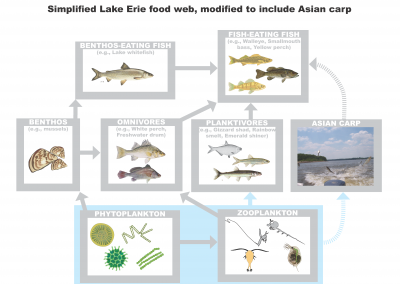
Simplified Lake Erie food web, modified to include invasive Carp. Invasive carp could affect the Lake Erie food web in two ways: by competing with native fish by eating their food and becoming food for other predatory fish. Graphic Credit: Hongyan Zhang, CIGLR Research Scientist.
Benthic Ecology Research Technician Andrew Camilleri uses a dissecting microscope to count and identify benthic (lake bottom) invertebrates including zebra and quagga mussels and Hexagenia (mayfly) larvae. Hexagenia are the preferred prey of many Great Lakes fish species. Their populations declined dramatically in the 1950s due to eutrophication, but recent evidence indicates that populations are now returning in some areas. This will likely have a positive impact on energy-flow pathways through the food web in areas where it becomes re-established. Photo Credit: Andrew Camilleri.
NOAA GLERL’S Research Scientist Ashley Elgin removing the settling plate from her Lake Michigan quagga mussel growth mooring. March 27, 2017. Photo Credit: Paul Glyshaw.
NOAA GLERL’S Research Scientist Ashley Elgin displaying the cages removed from her Lake Michigan quagga mussel growth mooring. March 27, 2017. Photo Credit: Paul Glyshaw.
Scientist entering the ballast tank of a bulk carrier vessel for collection of residual mud in the ballast tank to check for resting eggs of invasive species. T. Johengen, CILER Director. Photo Credit: D. Reid, GLERL.
Michigan Sea Grant’s El Lower sharing a food web game about Lake Huron. Photo Credit: Michele Wensman.
Video Library
Invasive carp have the potential to dominate the fish community and seriously devalue the vital recreational and commercial fisheries present there. Currently, CIGLR & our colleagues have produced models for Lake Erie & Lake Michigan that show what would happen if there were an invasive carp invasion and are working to develop similar ecosystem models for the other Great Lakes.
Video Library
Dmitry Beletsky is a Research Scientist at the Cooperative Institute for Great Lakes Research at the University of Michigan. His presentation explores the potential dispersal of Golden mussel (Limnoperna fortunei) larvae in Lake Michigan using a three-dimensional particle transport model.
Video Library
GLANSIS is an acronym for the Great Lakes Aquatic Nonindigenous Species Information System and is a “one-stop shop” for information about aquatic invaders in the Great Lakes region. It is a very collaborative inter-agency project and is a free tool for both scientists and the general public to learn more about what’s in their local waterways.